COVID-19/Flu-A/Flu-B Multiplex RT-PCR detection kit (Lyophilized)
Introduction
The New Coronavirus(COVID-19) is spreading all over the world. The clinical symptoms of COVID-19 and influenza virus infection are similar. So accurate detection and diagnosis of infected persons or carriers plays a vital role in the control of epidemic situation. CHKBio developed a kit that can that can simultaneous detect and distinguish COVID-19, Influenza A and Influenza B accurately. The kit also contains internal control to avoid false negative result.
Product Information
| Product Name | COVID-19/Flu-A/Flu-B Multiplex RT-PCR detection kit (Lyophilized) |
| Cat.No. | COV301 |
| Sample Extraction | One-step Method/Magnetic Bead Method |
| Sample Type | Alveolar lavage fluid, Throat swab and Nasal swab |
| Size | 50Test/kit |
| Internal Control | Endogenous housekeeping gene as internal control, which monitors the whole process of samples and tests, avoids false negatives |
| Targets | COVID-19, Influenza A and Influenza B as well as Internal control |
Product Features
Easy: All components are lyophilized, no need of PCR Mix setup step. Reagent can be directly used after dissolving, greatly simplifying the operation process.
Internal Control: monitoring process of operation and avoiding false negatives.
Stability: transported and stored at room temperature without cold chain,and it is verified that reagent can withstand 47℃ for 60 days.
Compatibility: be compatible with various real-time PCR instruments with four fluorescence channels in the market.
Multiplex: simultaneous detection of 4 targets including COVID-19, Influenza A and Influenza B as well as Internal control.
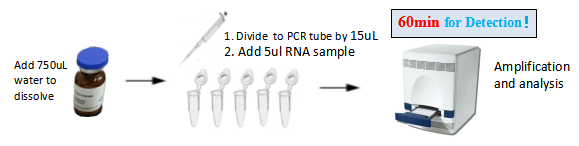
Detection Process
It can be can compatible with common real-time PCR instrument with four fluorescence channels and achieve accurate result.
Clinical Application
1. Provide pathogenic evidence for COVID-19, Influenza A or Influenza B infection.
2. Used for screening of suspected COVID-19 patients or high-risk contacts to give distinguishing diagnosis for COVID-19, Influenza A and Influenza B.
3.It is a valuable tool for evaluating the possibility of other respiratory infections( influenza A and influenza B) in order to carry out right clinical classification, isolation and treatment in time for COVID-19 patient.

 中文
中文