On February 11th, the Shanghai Chuangkun Biological TB-MDR-DNA detection kit was approved in Thailand. This is the third time that the Type 15 HPV typing detection PCR kit and TB-NTM PCR kit have been approved in 2023, and have obtained a registration certificate from the Thai FDA. While enriching Chuangkun's independent product line, Chuangkun Biotechnology has gained deep recognition from the Thai FDA, providing strong support for Chuangkun Biotechnology to further explore the international market.

According to WHO estimates, there will be 10.6 million new tuberculosis patients worldwide in 2022, with a incidence rate of 133/100000. In 2021, the estimated number of drug-resistant tuberculosis (MDR/RR-TB) cases will be 450000, an increase of 3% over 2020. This is the first reversal of the trend of annual decline of 3% or more since 2015. Among all new patients, 410000 (3.9%) are multi drug/rifampicin resistant tuberculosis patients, 3.3% of new patients are multi drug/rifampicin resistant, and 17% of retreated patients.
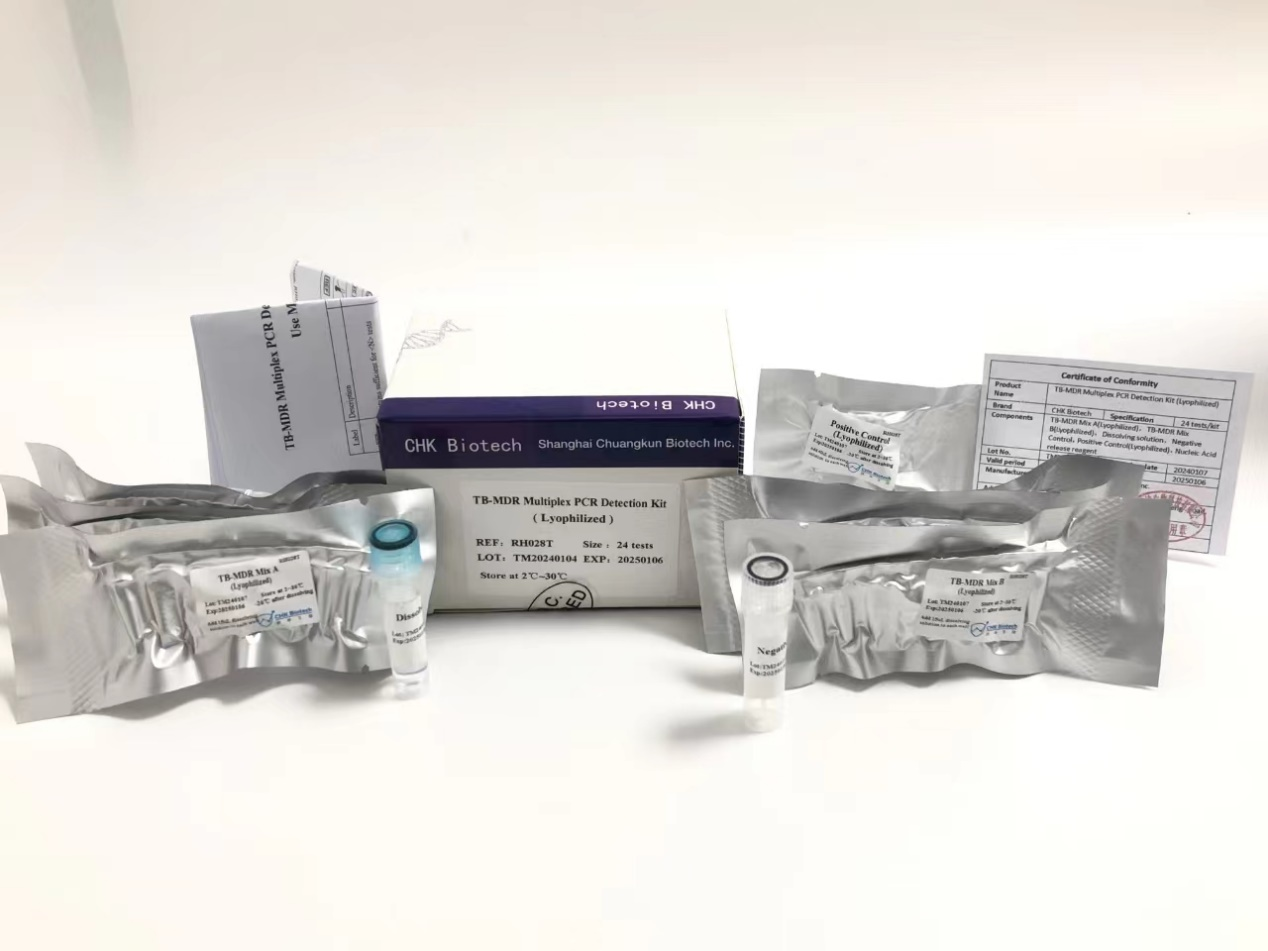
The TB-MDR-DNA detection kit independently developed and produced by Chuangkun Biotechnology can simultaneously detect 36 resistant sites of rifampicin and isoniazid. The innovative use of a full component freeze-drying process perfectly solves the drawbacks of traditional PCR reagent transportation. Compared to traditional tuberculosis detection, this reagent has advantages such as high sensitivity, strong specificity, convenient operation, and short time.
Obtaining the Thai FDA registration certificate again is a full recognition and affirmation of Chuangkun Biotechnology products. It will further enhance the popularity of Chuangkun products in the international market, and Chuangkun Biology will actively participate in the prevention and treatment of tuberculosis in the region.
Post time: Feb-23-2024

 中文
中文