Recently, Shanghai Chuangkun Biotechnology Co., Ltd. obtained the registration certificate of Thailand FDA for the 15 type HPV typing detection PCR kit, which indicates that Chuangkun Biotech’s products have been recognized by Thailand FDA, providing strong support for Chuangkun Biotech to further expand the international market.
According to the statistics of the World Health Organization, the incidence of cervical cancer in female malignant tumors worldwide is only second to lung cancer and breast cancer, ranking third. Every year, about 500000 women worldwide suffer from cervical cancer, and about 200000 women die of this disease. Cervical cancer is the only known cause of human malignant tumors. Studies have shown that human papilloma virus (HPV) infection is the main cause of cervical cancer and its precancerous lesions (cervical intraepithelial neoplasia (CIN)). On November 17, 2020, the World Health Organization (WHO) launched a global strategy to accelerate the elimination of cervical cancer, emphasizing the importance of HPV testing and screening. On July 6, 2021, WHO updated and released the guidelines for screening and treatment of cervical precancerous lesions in cervical cancer prevention, recommending human papillomavirus (HPV) DNA testing as the first screening method for cervical cancer screening.
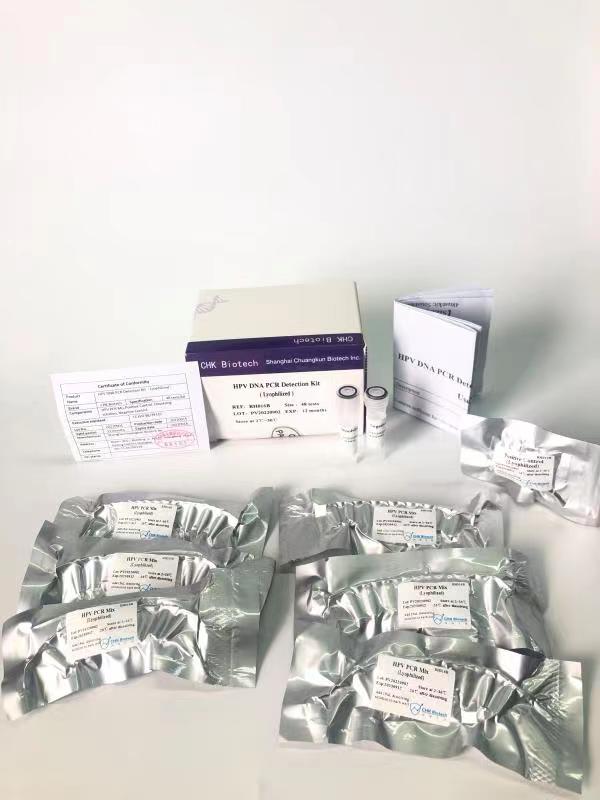
Chuangkun Biotech’s HPV nucleic acid typing test kit is based on multiple PCR fluorescence probe technology and is applicable to conventional four channel fluorescence quantitative PCR instrument. The product adopts the production process of full component freeze-drying. The kit can be transported and stored at room temperature, which solves the pain point of cold chain transportation for conventional liquid reagents, and can greatly reduce the logistics and transportation costs for overseas sales. This product is mainly used for in vitro detection of human papillomavirus in cervical exfoliated cells, covering 15 high-risk types, and specifically identifying 16 and 18 high-risk types. The product has the characteristics of high sensitivity (detection sensitivity up to 500 copies/ml), high specificity and high throughput. Using extraction free direct amplification technology and cooperating with the Thunder series rapid fluorescent PCR instrument detection equipment of Chuangkun Bio, the product can complete the rapid detection of 16~96 samples in 40 minutes, with accurate and reliable results.
Obtaining the registration certificate of Thailand’s FDA this time is a full recognition and affirmation of Chuangkun biological products. It will further enhance the popularity of Chuangkun’s products in the international market. In the future, Chuangkun will continue to adhere to the market orientation, take scientific and technological innovation as the support, continuously improve the core competitiveness of the enterprise, create a dominant brand with a global perspective, and strive to promote the development of the great health industry and realize the health dream of mankind through unremitting efforts and persistence!
Post time: Jan-05-2023

 中文
中文