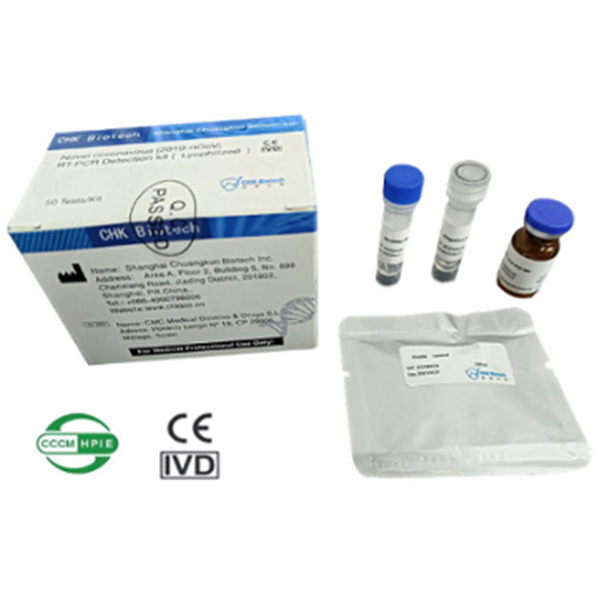
Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit (Lyophilized)
Introduction
Novel Coronavirus(COVID-19) belongs to β genus coronavirus and is a positive single strand RNA virus with a diameter of about 80-120nm. COVID-19 is an acute respiratory infectious disease. People are generally susceptible to COVID-19. Asymptomatic infected persons may also be the source of infection. Novel Coronavirus (2019-nCoV)RT-PCR Detection Kit (Lyophilized ) developed by CHKBio can be transported and stored at room temperature, which can greatly help the global fight against epidemic.
Product Information
| Product Name | Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit (Lyophilized ) |
| Cat.No. | COV001 |
| Sample Extraction | One-step Method/Magnetic Bead Method |
| Sample Type | Alveolar lavage fluid, Throat swab and Nasal swab |
| Size | 50Test/kit |
| Internal Control | Endogenous housekeeping gene as internal control, which monitors the whole process of samples and tests, avoids false negatives |
| Targets | ORF1ab gene,N gene and Internal control gene |
Product Features
Easy: All components are lyophilized, no need of PCR Mix setup step. Reagent can be directly used after dissolving, greatly simplifying the operation process.
Internal Control: monitoring process of operation and avoiding false negatives.
Stability: transported and stored at room temperature without cold chain,and it is verified that reagent can withstand 47℃ for 60 days.
Compatibility: be compatible with various fluorescent PCR platforms, including conventional PCR machines and micro-chip fast PCR machines(UF-300).
Multiplex: simultaneous detection of 3 targets including ORF1ab gene,N gene and Internal control gene.
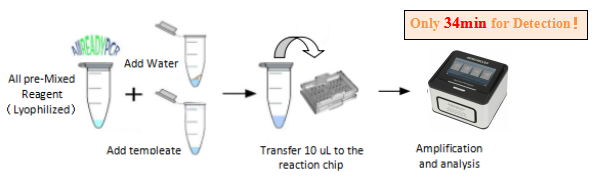
Detection Process
(1) With common fluorescent quantitative PCR instrument is achieving accurate detection.
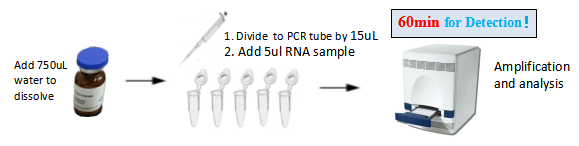
(2) It also can be used with mobile molecular POCT platform of our company for on-site real-time screening.
Clinical Application
1. Provide pathogenic direct evidence for COVID-19 infection.
2. Used for screening of suspected COVID-19 patients or high-risk contacts.
3.It is a valuable tool for evaluating the curative effect and clinical rehabilitation.

 中文
中文